Interfacial tension is somewhat similar to surface tension in that cohesive forces are also involved. However the main forces involved in interfacial tension are adhesive forces (tension) between the liquid phase of one substance and either a solid, liquid or gas phase of another substance. The interaction occurs at the surfaces of the substances involved, that is at their interfaces.
(i) Spreading parameter
Let's focus our attention on the case of two non-miscible liquids A and B floating on one another. The liquid substrate B (water in following demo) is assumed to be denser. The liquid A (soap solution) is laid down on the top.
Starting with the three surface and interfacial tensions γA,γB,γAB, the spreading parameter (S) can be determined:
S=γB - (γA+γAB)
If S is positive, wetting is total and the liquid spreads completely. Such is the case in the following demonstration for soap solution (25 mN/m) over water (72 mN/m).
Click here for the video
Lycopodium powder is sprinkled into a container of water. The powder floats evenly separated on the surface of the water. When liquid detergent is dropped into the water the surface tension at this place decreases. The surface wants to expand and the powder is pushed to the rim. Click here to find more about Benjamin Franklin's experiment of oil spreading on water , which could be used to get an estimate of the size of a molecule.
(ii) Emulsification
Interfacial tension also plays a important role in emulsification which is the process of preparing emulsions, which are heterogeneous systems consisting of at least one immiscible liquid intimately dispersed in another in the form of droplets.
If two immiscible liquids are in contact with each other, they will tend to maintain as small an interface as possible (as interfacial energy is associated with formation of any interfacial area). Consequently, it is very difficult to mix the two liquids. When they are shaken together, spherical droplets will form, as the liquids tend to maintain as small a surface area as possible and an interfacial tension will be maintained between the two liquids. When a “surface active” ingredient is added, its molecules will tend to be oriented between the two faces with the
polar ends in the polar phase and the non polar ends in the non polar phase, which will lower interfacial tension. This will result in miscibility of the two liquids.
Salad Dressing
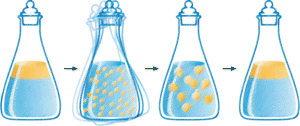
Click here for detailed discussion.
Photo courtesy: http://www.chromatography.amershambiosciences.com/
Consider what happens when you prepare a salad dressing. You basically mix oil, water and herbs. When you shake this mixture a lot of small droplets of oil are formed and suspended in the water. If you have done your shaking well the suspension will last for some time, but will eventually go back to the state with two separate phases, one water phase and one oil phase. If you follow this return to two separate phases closely you will notice that it starts with the joining of small droplets to form larger and larger ones.
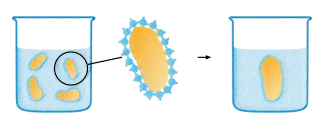
Photo courtesy: http://www.chromatography.amershambiosciences.com/
When a hydrophobic substance is immersed in water something analogous to the surface tension phenomenon happens. The water molecules cannot "wet" the surface of the hydrophobic substance. Instead they will form a highly ordered shell, around the hydrophobic substance, caused by the lack of possibility to form hydrogen bonds in all directions. Now, minimizing the extent of this shell will lead to a decrease in the number of ordered water molecules which represents a thermodynamically more favorable situation by an increase in entropy (DS). Consequently hydrophobic surfaces combine to accomplish this. We know have an explanation for oil droplets combining in the dressing.
|














